Every single second inside every living cell, thousands of chemical reactions are taking place. These reactions are performed by enzymes.
Enzyme
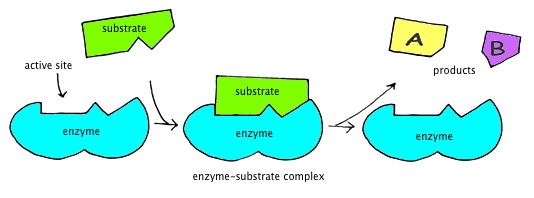
An enzyme is actually a protein which helps to catalyze a chemical reaction. It initiates the reaction, speeds up the reactions progress, and makes sure the outcome is always the same. These enzymes often work together to form longer pathways, such as the Citric Acid Cycle, which is a series of chemical reactions used by cells to generate energy from carbohydrates.

The essential tasks of life such as metabolism, protein synthesis, and cell renewal and growth are all regulated by enzymes. The life-sustaining power of enzymes lies in the fact that they catalyze reactions in mild conditions of pH, temperature, and atmospheric pressure. The rates of catalyzed reactions are millions to trillions times faster than those of the same reactions un-catalyzed.
To speed up a reaction in the absence of enzymes, additional energy would need to be provided as heat, which is jostles the substrates and occasionally provides enough energy to trigger a reaction.
In the course of most reactions, an unstable and highly energetic transition state is formed as the substrates are transformed into products. An enzyme acts as a template for the reaction, binding to its substrate and holding it in the proper position to form the product. An enzyme also surrounds the substrate with reactive groups that stabilize the transition state, making it easier for the reaction to occur.
How Do They Work?
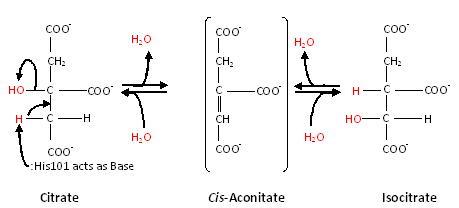
To understand how enzymes work, let's discuss the reaction in the Citric Acid Cycle that is catalyzed by the enzyme Aconitase. Aconitase binds to its substrate citrate and removes a hydroxyl group and a hydrogen atom to form intermediate Cis-aconitate.
It then adds the hydrogen in the hydroxyl back in slightly different positions to form the product Isocitrate.
In the active site, some amino acids are perfectly positioned to recognize the substrate and hold it in the optimal position for the reaction to begin. Some amino acids are involved in recognizing and holding the substrate. Other amino acids are directly involved in catalysis.
Histidine 101 acts as an acid by donating its proton. Thanks to the chemical environment of Serine 642, it can act as a base by accepting the proton from the substrate. The active site of Aconitase also contains an iron-sulfur cluster that stabilizes the substrate electrostatically and helps to position it relative to the catalytic residues.
The first step in the reaction is dehydration. In this step, Histidine acts as an acid and protonates the hydroxyl on the substrate allowing it to leave as a water molecule. Serine then acts as a base by extracting a hydrogen atom from the substrate's opposite side, forming the intermediate Cis-aconitate. Cis-aconitate is then turned upside down, and the complementary hydration reaction occurs.
In this step, Histidine grabs a hydrogen atom from a passing water molecule, placing the resulting hydroxyl group back onto the substrate. Serine then returns its hydrogen atom, resulting in the final product Isocitrate.
Notice that the enzyme itself was not changed by the reaction. It extracted the hydroxyl group and a hydrogen atom and then put them back, starting and ending in the same state. This is the hallmark of a catalyst, when it finishes a reaction, it is ready for the next, so it can perform thousands of reactions in a row.
Notice also that the shape of an active site is often flexible. Many enzymes surround their substrates, closing around them to form the perfect environment for a reaction.
Enzymes are fundamental to life on Earth, working every second of every day to maintain life processes in every cell, and in every living creature on the planet.