Pretty much everyone knows with this is even if they don't know much about chemistry. The periodic table of the elements, which at first glance appears to be a haphazard collection of substances, most of which sound weird and alien, but when the components are ordered, many lovely patterns emerge, revealing much about how nature functions.
Periodic Table
 In the mid 1800's lots of chemists were trying to come up with a way to depict all the elements in table form and many different formats were proposed but it was the one by Dmitri Mendeleev that has endured due to the accuracy with
which it links data and the predictability of its results.
In the mid 1800's lots of chemists were trying to come up with a way to depict all the elements in table form and many different formats were proposed but it was the one by Dmitri Mendeleev that has endured due to the accuracy with
which it links data and the predictability of its results.
He arranged the elements into rows called periods and columns called groups elements, that had similar behavior were put in groups together, which aided in the correlation of pre-existing data and indicated the existence of unseen components.
Mendeleev predicted that certain properties of the elements
that would fill in the table's gaps would eventually be discovered, and we now
have a neat arrangement of all the metals, metalloids, and nonmetals. At the
time, it wasn't known, but the reason elements in the same group behave
similarly is because they share the same number of valence electrons.
Look at group 1, for example, these elements all have one valence electron or one electron in their outermost shell. As you go down the table and N increases, you gain a shell each time but whichever is the outermost shell there is only one electron in it.
Every element in group 2 has two electrons in its outermost shell and so forth this simple fact determines many characteristics about each element.
Periodic Trends
There are some periodic trends that we can recognize when we look at the table.
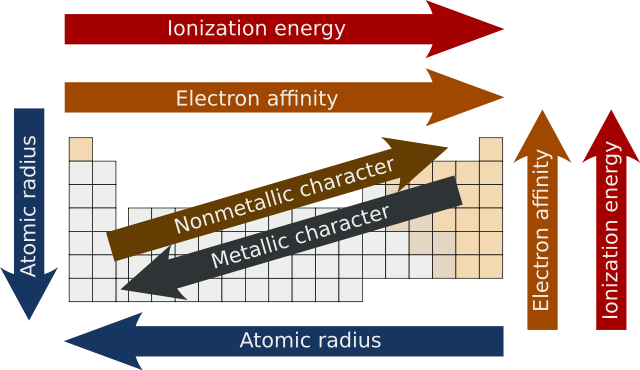
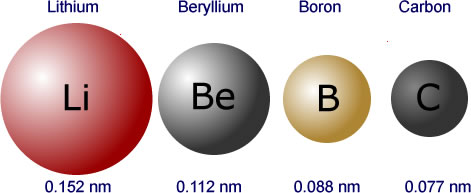
The first one is atomic radius or the size of the atom. Atomic size grows as we move down the table due to the addition of shells. As we go to the right, atomic radius decreases because we are moving within a shell and each element to the right has one more proton in the nucleus than the last. The electrons have a stronger electromagnetic pull as a result, and when the radius decreases, the overall atomic radius on the periodic table rises.
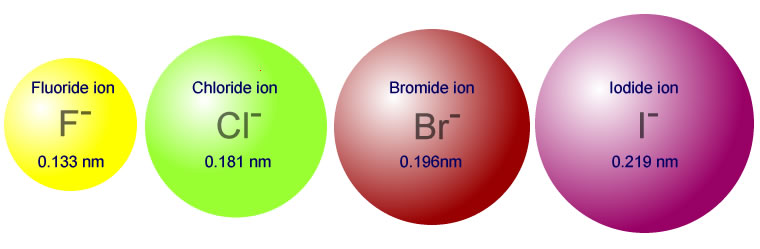
Ionic radius is a little different. Electrons repel each other so adding an electron makes an atom bigger. Taking one away, makes it smaller. Ions with the same electron configuration will have their radii decrease as the atomic number increases.
The energy needed to remove an electron from an atom is known as Ionization energy. It will always be an electron in the outermost shell. With distance, the electromagnetic force that pulls electrons toward protons rapidly diminishes. So the farther away an electron is from the nucleus. The easier it is to pull it away. This means the ionization energy trend is precisely the opposite of the atomic radius trend.
Francium, a very large atom with only one valence electron will be easy to ionize because the electron is so far away from the nucleus and atoms like to have their outermost shell completely full losing the electron. Means this shell is gone and the one below is completely full. So elements in group 1 will easily lose one electron.
Looking at the opposite corner with Helium, there is only one shell. So the electrons are very close to the nucleus, and the shell is full so it is very stable. For this reason, it requires much more energy to ionize helium. So the ionization energy increases going this way on the periodic table. Elements can have successive ionization energies for removing more than one electron. A second ionization energy will always be greater than the first and continue to increase from there, since the more electrons you remove the less stable the atom becomes. An element will have a huge jump in ionization energy after you take the last one in a shell. Because then you jump to the noble gas electron configuration from the previous shell which is full. So it really won't want to give up any more electrons.

There are just a couple exceptions to the ionization energy trend but we can rationalize them. Look for example at the second row from Lithium to Neon. The ionization energy should increase each time we add a proton to the nucleus and the radius contracts a little bit. But something like Oxygen which dips downwards from Nitrogen's ionization energy does so because of orbital symmetry.
Here is Nitrogen's orbital diagram as well as oxygen's.

Notice that nitrogen's 2p orbitals are precisely half full. This gives nitrogen a special stability, just like elements that have a full outermost shell. If Nitrogen loses an electron, it loses that special stability. But if Oxygen loses an electron, it will gain that special stability. That's why Oxygen's ionization energy is a little bit lower than Nitrogen's. Even though Oxygen has one additional proton.
Next we will look at electron affinity. This is exactly the opposite of ionization energy. Since ionization energy is how much energy you need to remove an electron and electron affinity tells us how much an atom wants to gain an electron. Disregarding the noble gases as their shells are full, electron affinity increases this way.
Fluorine has the highest electron affinity because if it gains one electron it will have a full shell or noble gas electron configuration. Looking at the opposite corner, these elements don't want to gain electrons. They would rather lose them exceptions to this trend happen for exactly the same reasons as the exceptions to the ionization energy trend.
Lastly we want to look at electronegativity, this is the ability of an atom to hold electrons tightly. It will increase this way, because a smaller atom like Fluorine with more protons for its energy level or higher effective nuclear charge will hold electrons best. Again we will disregard the noble gases for this trend.