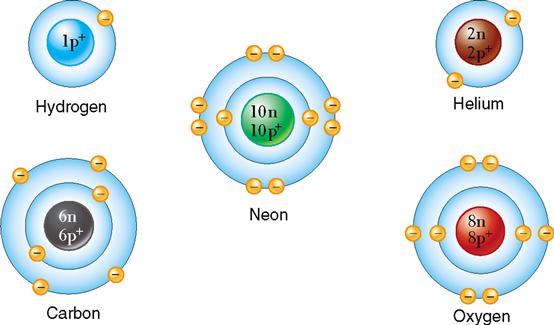
The fundamental unit of chemical elements is the atom. An atom contain subatomic particles in their nucleus and in the electron cloud that surrounds the nucleus.
These subatomic particles include: Protons and Neutrons, which are located in the nucleus, and electrons, which are located in the cloud, or field that surrounds the nucleus.
In chemical reactions between atoms, only the electrons in the outermost shell participate in the resulting chemical bond.
In atoms with only one energy level or shell, 2 electrons are required for stability. If we look at helium, which has only 1 shell or energy level, we see that it is full, because it contains 2 electrons. So helium is stable, and there is no naturally occurring compound containing helium.
Hydrogen, which also has only one ring or shell, possesses only one electron in its shell, so it is chemically reactive. In atoms with more than one energy level or shell, 8 electrons are required in its outermost shell for stability.
So, neon, which has 8 electrons in its outermost shell, is stable, and oxygen, which has only 6 electrons in its outmost shell, is chemically reactive.
Atoms that do have fewer than 8 electrons
in their outermost shells, will try to share, gain, or lose electrons in order
to become stable. This is called the octet rule, but there are some exceptions,
such as we saw with hydrogen and helium which only have one energy level or
shell. And that, be the basics on atomic structure.