
Immunohistochemistry (IHC) is a powerful laboratory technique that plays a crucial role in the field of biology and medicine. It enables researchers and clinicians to visualize specific proteins within cells and tissues. IHC is widely used to understand disease mechanisms, diagnose illnesses, and guide treatment decisions. In this essay, we explore the principles, applications, and significance of immunohistochemistry in the realm of life sciences.
Immunohistochemistry is a laboratory method used to detect specific proteins in biological tissues. It combines principles of immunology with histology to precisely locate and quantify proteins within cells and tissues. It is a fundamental tool in cellular and molecular biology research and clinical diagnostics.
Principles of Immunohistochemistry
- The core of IHC is the highly specific binding between antibodies and target proteins. Antibodies are designed to recognize and bind to a particular protein or antigen.
- To enhance the detection of target proteins, antigen retrieval techniques are used to unmask and expose antigens in formalin-fixed tissues.
- Secondary antibodies are utilized to amplify the signal. They recognize and bind to the primary antibody, often tagged with a detectable marker.
- The target protein-antibody complexes are visualized using various methods, such as enzyme-based chromogenic reactions or fluorescent markers.
Steps Of Immunohistochemistry Test
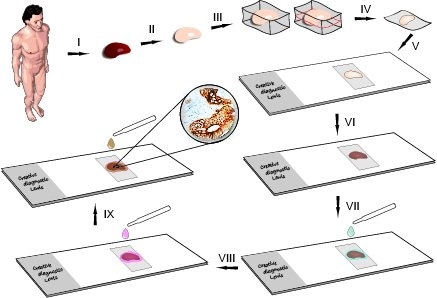
1. Tissue Collection and Fixation:
Collect the tissue sample of interest.
Fix the tissue using a suitable fixative (e.g.,
formaldehyde) to preserve cellular structures.
2. Tissue Processing:
Dehydrate the tissue through a series of graded alcohols to remove water. Embed the tissue in a suitable embedding medium (e.g., paraffin) for sectioning.
3. Sectioning:
Cut thin sections (usually 4-5 micrometers thick) of the
embedded tissue using a microtome.
4. Mounting:
Mount the tissue sections onto glass slides.
5. Deparaffinization:
If paraffin-embedded, deparaffinize the sections using
xylene or other clearing agents.
6. Rehydration:
Rehydrate the sections through a series of graded alcohols
to restore water content.
7. Antigen Retrieval:
Perform antigen retrieval if necessary to expose antigens
masked during fixation. This can be done using heat (e.g., in a pressure
cooker) or enzymatic methods.
8. Blocking:
Block non-specific binding sites by incubating the sections
in a blocking solution containing proteins like bovine serum albumin (BSA) or
normal serum.
9. Primary Antibody Incubation:
Incubate the tissue sections with a primary antibody
specific to the target antigen. The primary antibody binds to the antigen of
interest.
10. Washing:
Wash away unbound primary antibodies and blocking reagents
to reduce background staining.
11. Secondary Antibody Incubation:
Incubate the sections with a secondary antibody conjugated
to a detection molecule (e.g., enzyme or fluorophore). The secondary antibody
recognizes the primary antibody and amplifies the signal.
12. Washing:
Wash away unbound secondary antibodies.
13. Signal Detection:
If using an enzyme-conjugated secondary antibody, add a
substrate that reacts with the enzyme to produce a visible signal. If using a
fluorophore-conjugated secondary antibody, proceed to the next step.
14. Counterstaining (Optional):
Counterstain the tissue with a dye (e.g., hematoxylin) to
visualize cellular structures.
15. Mounting Coverslips:
Apply a mounting medium and cover the tissue sections with a
coverslip.
16. Microscopy:
Examine the stained tissue sections under a microscope.
17. Image Analysis:
Capture and analyze images to assess the expression and
localization of the target antigen.
It's important to note that variations in protocols may exist based on the specific antibodies, tissues, and detection methods used. Optimization may be necessary for individual experiments. Always refer to the manufacturer's instructions for the specific antibodies and reagents you are using
Applications of Immunohistochemistry
- IHC is commonly used to diagnose cancer by identifying specific tumor markers. It aids in determining cancer type, stage, and prognosis.
- IHC is crucial for studying brain proteins and neurotransmitters. It helps in understanding brain function and disorders like Alzheimer's and Parkinson's disease.
- IHC assists in the identification of pathogens and their proteins in infected tissues, contributing to the diagnosis and study of infectious diseases.
- In autoimmune diseases, IHC helps identify autoantibodies and autoimmune-related proteins.
- IHC is used to track specific protein markers associated with stem cells and their differentiation.
Significance of Immunohistochemistry
- IHC plays a significant role in personalized medicine. By identifying specific proteins, clinicians can tailor treatments to individual patients.
- IHC is indispensable in advancing our understanding of various diseases and their underlying molecular mechanisms.
- In pathology and clinical labs, IHC is essential for diagnosing diseases, guiding treatment decisions, and predicting patient outcomes.
Challenges and Future Trends
Challenges in IHC include issues with antibody specificity and variability in sample quality. Future trends involve the development of more robust antibodies, automated systems, and the integration of IHC with other omics technologies, such as genomics and proteomics.
Conclusion
Immunohistochemistry is a powerful tool that bridges the gap between molecular biology and histology. It enables researchers and healthcare professionals to visualize and understand the distribution of specific proteins within tissues. Its applications in medical diagnosis, research, and personalized medicine make it an invaluable asset in the field of life sciences, offering new insights and opportunities for better patient care and scientific discovery.
