You have likely produced ammonia in the laboratory from the thermal decomposition of ammonium chloride, or from the reaction of ammonium and hydroxide ions.
Have you ever wondered how ammonia is produced on an industrial scale?
In this article, I will discuss about the Haber-Bosch process to produce ammonia, developed during World War I by Fritz Haber and Carl Bosch. For their efforts and contributions to developing large-scale industrial processes, they were awarded with Nobel Prizes in Chemistry.
The Haber-Bosch process is the industrial process for the manufacture of ammonia from hydrogen and nitrogen.
Hydrogen is obtained from the reaction of methane and steam, producing carbon monoxide as a byproduct. The hydrogen produced from this reaction also reacts with oxygen from air, producing water, and leaving nitrogen behind. Recall that air is 77% nitrogen. These gases are then compressed and delivered to the reactor where ammonia is produced. These gases are then cooled down, and ammonia is liquefied, ready to be tapped off. Unused hydrogen and nitrogen are recycled back to the reactor.
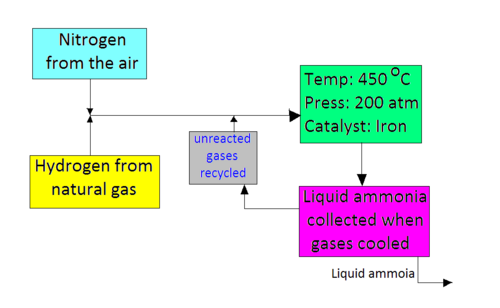
Note that this is a reversible reaction and that the forward reaction is exothermic.
.png)
The Haber-Bosch process uses Le Chatelier's principle to maximize ammonia production while keeping operating and production costs in mind. Le Chatelier's principle tells us that increasing pressure will favor the side with fewer moles, for our case, the production of ammonia. It would make sense to conduct the reaction at a very high pressure, but we must also remember that it is expensive to build and operate a plant that can withstand such high pressures. Therefore, a compromise pressure of 200atm is used.
Keeping Le Chatelier's principle in mind, what do you think is the best temperature conditions to maximize ammonia production?
Since the forward reaction is exothermic, it would make sense to conduct this reaction at a low temperature. The compromise temperature is 400-450¼C Ð which is not exactly low, but not too high. If a low temperature were used, the rate of reaction would be very slow, though the exothermic reaction would be favored. It would also actually take a long time for equilibrium to be reached, so a compromise temperature of 400-450¼C ensures that the reaction proceeds with sufficient yield. The yield of this process is 10-20% - remember from the introduction that unused gases are recycled, so no reactants are wasted. As well, an iron catalyst is used in this process. The presence of the catalyst does not affect the position of equilibrium, but increases the rate at which equilibrium is reached.
Conclusion
The Haber-Bosch process is the industrial process to produce ammonia from hydrogen and nitrogen. It is conducted at 400-450¼C and at 200atm in the presence of an iron catalyst. These are compromise conditions in order to maximize ammonia production and economic profit.
